Unlike ionic compounds they do not dissolve in water nor do they conduct electricity. These compounds are also reported exhibiting higher melting points and densities These are also called as saturated hydrocarbons since saturated compounds form ring structure Since the electronegativity between the carbon-hydrogen bonds is found to be too less for these compounds they are said to be not having any polarity between the bonds.

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download
They are very hard somewhat brittle solids with extremely high melting points higher than 1000 C or 1800 F.


Naming Ionic Compounds A Guided Inquiry Exercise
Naming Binary Covalent Compounds Ppt Video Online Download

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Solved That They Do Part I Nomenclature Of Binary Ionic Chegg Com
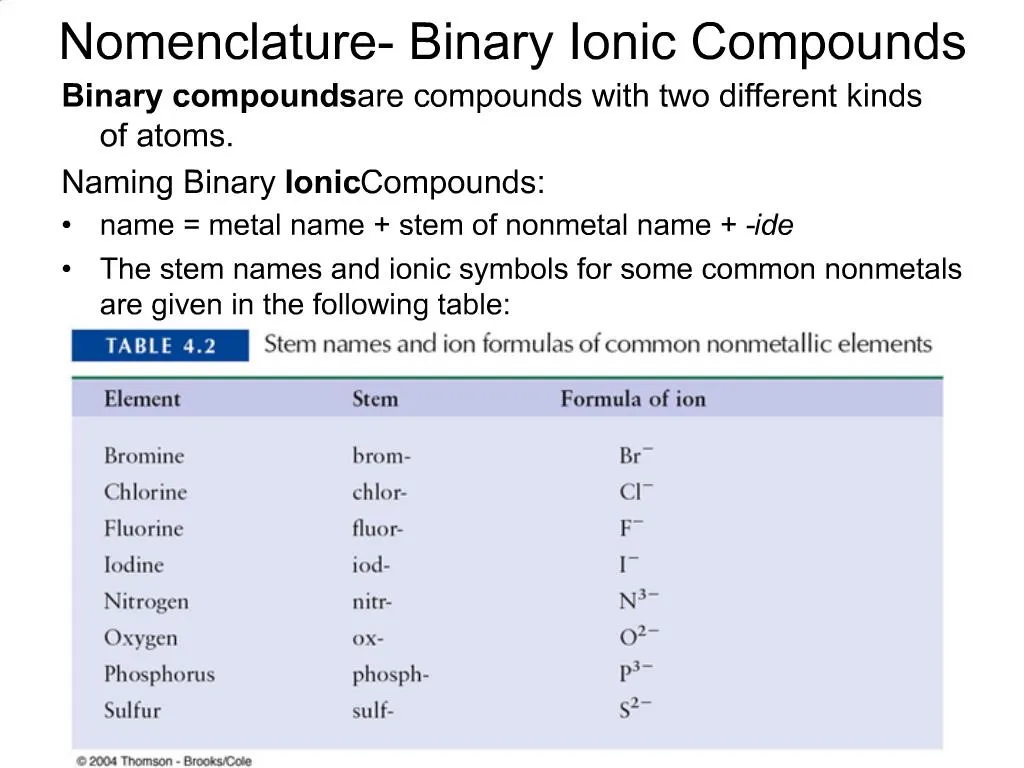
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132

Naming And Writing Formulas For Binary Ionic Compunds Panduan Kimia Riset
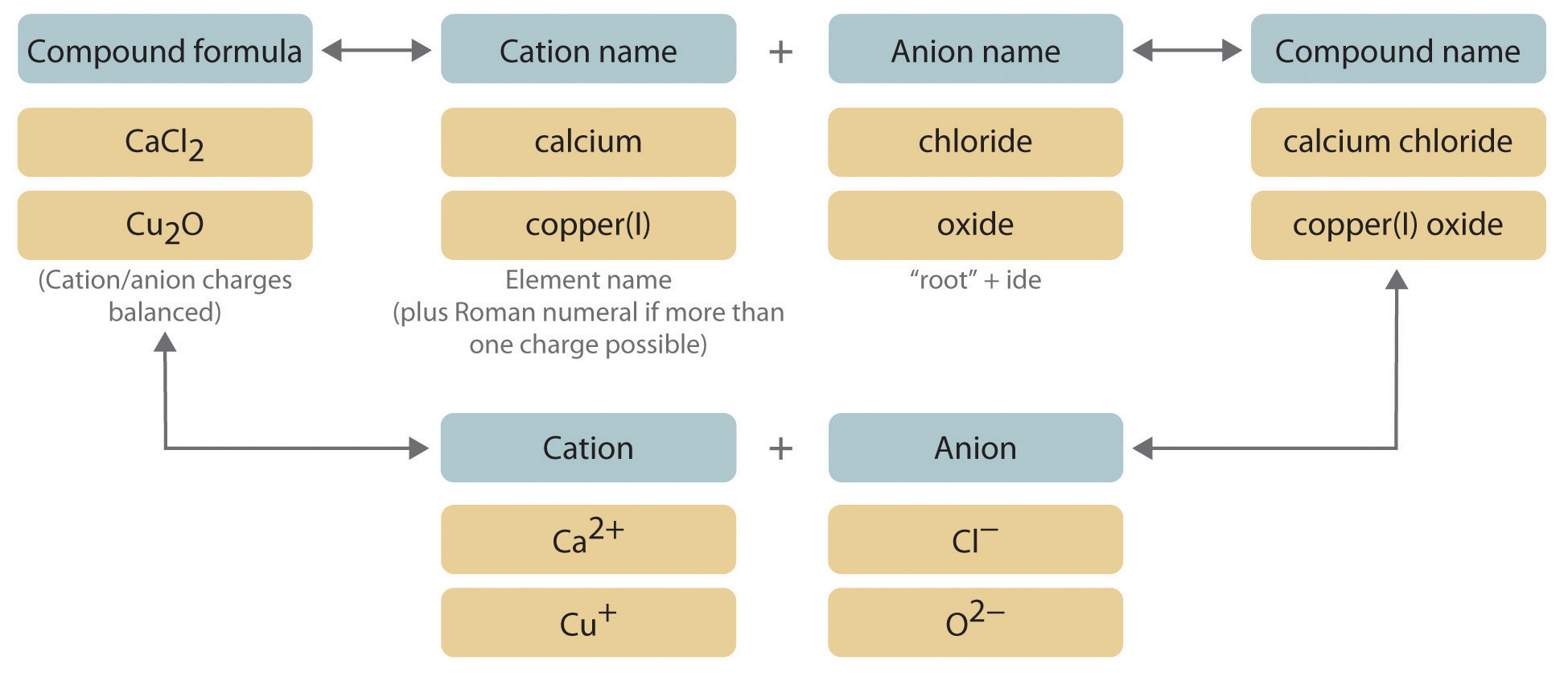

0 comments
Post a Comment